 |
Product Information
Description: BioLon™ is a sterile, nonpyrogenic, optically clear, viscoelastic preparation of highly purified, high molecular weight sodium hyaluronate. BioLon™ contains 10 mg/ml of sodium hyaluronate dissolved in a physiological sodium chloride phosphate buffer (pH 6.8 - 7.6). This high molecular weight polymer is made up of repeating disaccharide units of N-acetyl-glucosamine and sodium glucuronate linked by (beta)-1,3 and (beta)-1,4 glycosidic bonds.
Sodium hyaluronate is a physiological material that is widely distributed in the connective tissues of both animals and man. Chemically identical in all species, hyaluronate can be found in the vitreous and aqueous humor of the eye, the synovial fluid, the skin and the umbilical cord.
BioLon™ has the following properties:
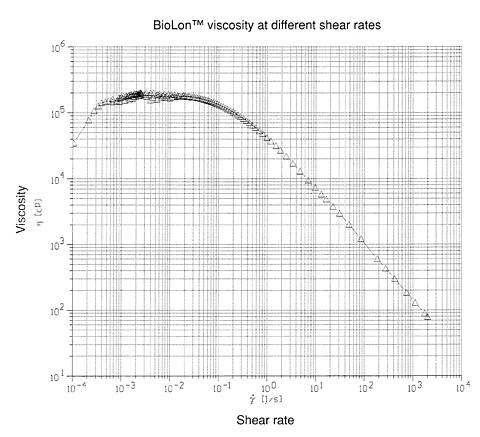
Each milliliter of BioLon™ contains: sodium hyaluronate, 10 mg; sodium chloride, 8.5 mg; disodium hydrogen phosphate dodecahydrate 0.56 mg; sodium dihydrogen phosphate dihydrate 0.045 mg; water for injection q.s. BioLon™ has an osmolality of 260-380 mOsm/kg and a viscosity of approximately 100,000 cps at a shear rate of 0.1 sec -1 (25°C).
 |
Indications: BioLon™ is indicated for use as a surgical aid to protect corneal endothelium during cataract extraction (extra-capsular) procedures, intraocular lens (IOL) implantation and anterior segment surgery. When introduced in the anterior segment of the eye during these surgical procedures, BioLon™ serves to maintain a deep anterior chamber.
In addition, BioLon™ helps to push back the vitreous face and prevent formation of a post-operative flat chamber.
Contraindications: When used as recommended there are no known contraindications to the use of BioLon™.
Warnings: Mixing of quaternary ammonium salts such as benzalkonium chloride with sodium hyaluronate results in the formation of a precipitate. The eye should not be irrigated with any solution containing benzylalkonium chloride if BioLon™ is to be used during surgery.
Adverse Reactions: In clinical trials, 298 patients were treated with BioLon™ and 224 patients were treated with sodium hyaluronate, an approved comparative device on the U.S. market for more than five years. The incidences of adverse experiences that were reported in >1% of the patients are shown in Table I.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Adverse events which occurred in <1% and in at least 2 patients include: ocular hemorrhage, corneoscleral leak, suture related adverse events, vitreous in anterior chamber, hyphema and hematic Tyndall, synechiae, capsule rupture, and cyclytic membrane.
Dosage/Applications: Cataract Surgery and Intraocular Lens Implantation
Refrigerated BioLon™ should be allowed to attain room temperature (approximately 20 - 30 minutes) prior to use. The usual dose required is 0.2 to 0.5 ml of BioLon™. BioLon™ should be slowly and carefully introduced into the anterior segment of the eye using the provided cannula.
Injection of BioLon™ can be performed either before or after delivery of the lens. BioLon™ may also be used to cost surgical instruments and the intraocular lenses prior to insertion.
Additional BioLon™ can be injected during surgery to replace any BioLon™ lost during surgical manipulation (see Precautions section).
How Supplied: BioLon™ is supplied in sterile, disposable 2.25 ml syringes containing either 0.5 ml or 1.0 ml of 1% sodium hyaluronate in phosphate-buffered salt solution. Each product package contains a blister-packed syringe, a sterile, single-use ophthalmic cannula (anterior chamber irrigator) and a package insert.
Storage Instructions: Store in a cold dark place (2° - 8°C; 36° - 48°F). May be kept at 25°C (77°F) for up to one month. Protect from freezing. Bring to room temperature prior to use.
Caution: Federal law restricts this device to sale by or on the order of a physician.
Manufactured by: Bio-Technology General, (Israel) Ltd., Kiryat Weizmann, Rehovot 76326, Israel
Manufactured for: Akorn, Inc., Buffalo Grove, Illinois 60089
 |
 |
 |
 |
 |
1812-5020
9/98